
Mixolifuse is formulated to support healthy levels of hair enzymes, which helps minoxidil work effectively to promote hair growth 1-4


Mixolifuse is formulated to support healthy levels of hair enzymes, which helps minoxidil work effectively to promote hair growth 1-4

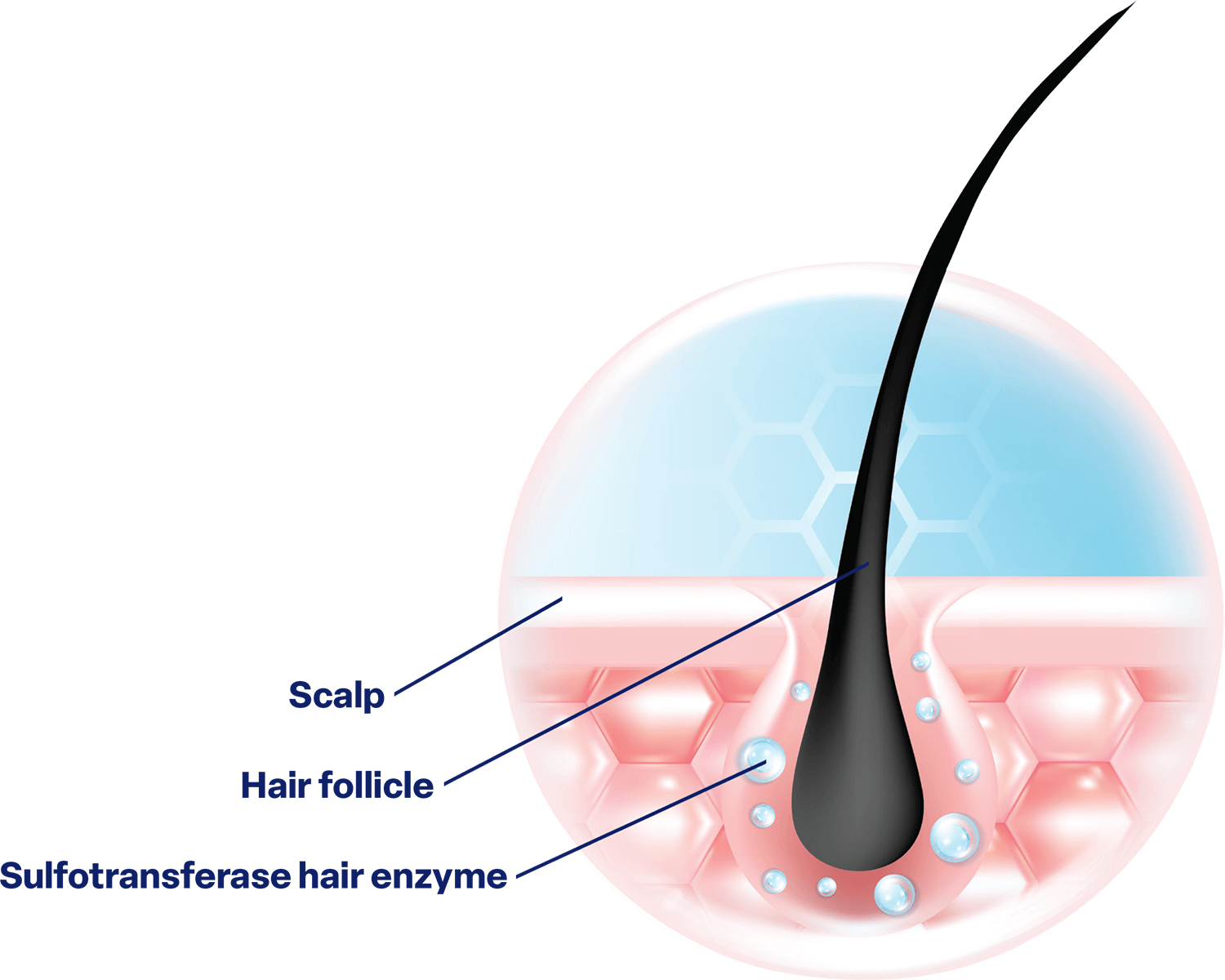
Sulfotransferase is an important enzyme found in the scalp that is needed for healthy hair growth 2,3,5

STA = sulfotransferase activity. *As measured by Optical Density (OD; 'Lower' ≤OD405<0.4, 'Higher' ≥ OD405>0.4), the conversion of minoxidil to minoxidil sulfate is coupled to the conversion of p-nitrophenyl sulfate to p-nitrophenyl, which can be quantified by optical absorbance at 405 nm
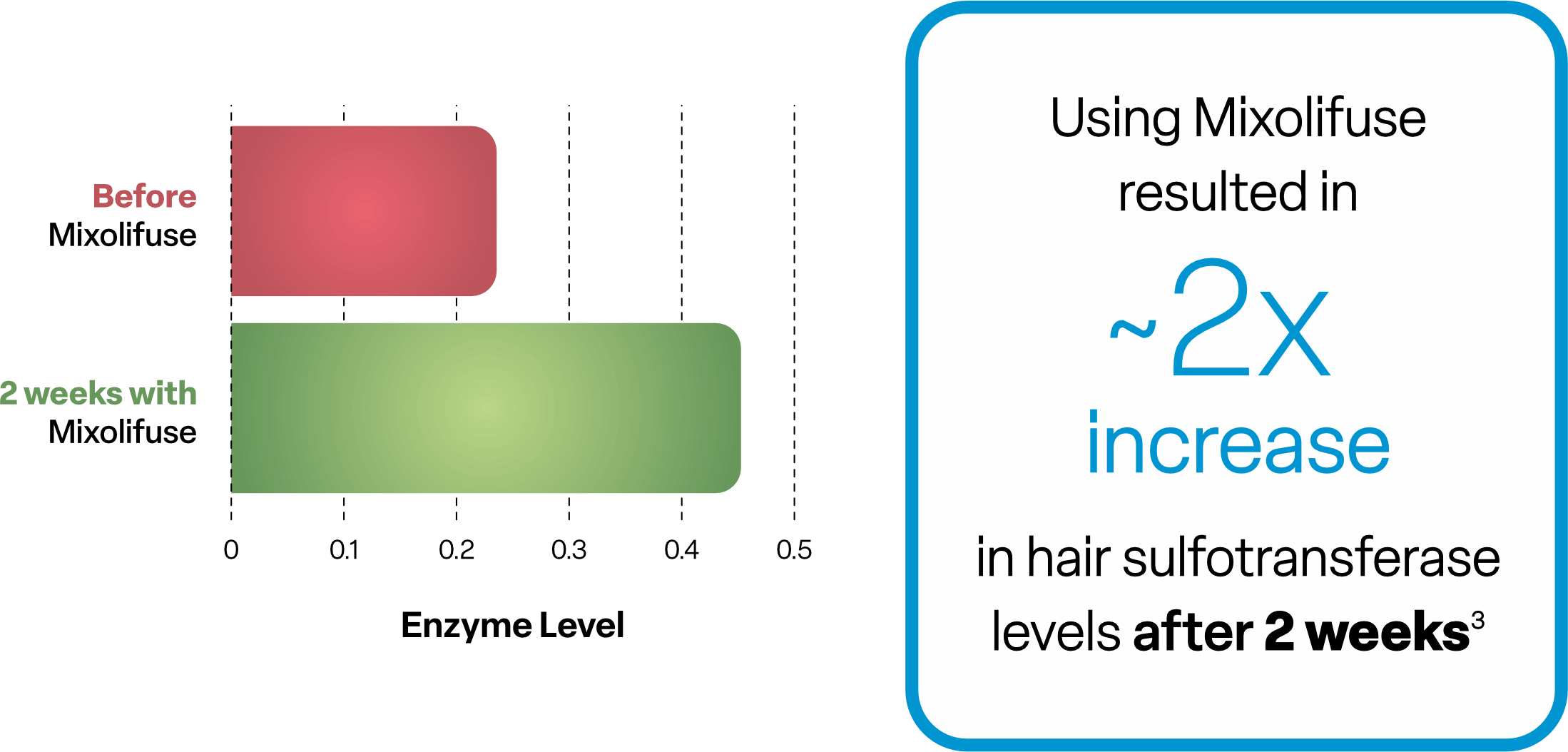

Mixolifuse is a topical treatment specially formulated to enrich sulfotransferase activity 2-4
Mixolifuse is a lightweight topical spray designed to easily integrate into a daily minoxidil hair growth routine1



Australian distributor for Mixolifuse is:
Glenmark Pharmaceuticals Australia Pty Limited
Suite 1503 Level 15, 14 Martin Place,
Sydney, NSW 2000, Australia.
For more information about Glenmark Pharmaceuticals, please visit: www.glenmark.com.au
Ready to buy Mixolifuse?
Contact your Pharmacy.
For Retail Pharmacists:
Interested to stock Mixolifuse?
Call Pharmabrokers on (02) 8878 9733
Local Contact:
Michael Moffitt, Country Manager
Australia & New Zealand
Telephone No.: (+61) 0412 387 087
Email:
Michael.Moffitt@glenmarkpharma.com
Medical Information, Product Quality and Adverse Event Enquiries:
Telephone No.: 1800 371 274
Email:
Medinfo.Australia@glenmarkpharma.com
References: 1. Dhurat R et al. J Cosmet Dermatol 2022;21(1):343–346. 2. Pietrauszka K, Bergler-Czop B. Adv Dermatol Allergol 2022;39(3):472–478. 3. Ramos PM et al. J Eur Acad Dermatol Venereol 2020;34(12):e799–e800. 4. Goren A et al. Dermatol Ther 2014;27(3):171–3. 5. Cortez GL et al. An Bras Dermatol 2025;100(2):308–321.
GLE0020; Date of preparation: November 2025. G-INPLMS32360.